Water-Free Proton Conduction in Hexakis(p-Phosphonatophenyl)benzene
Hydrogen has a high potential as energy carrier in the field of renewable and clean energy. The conversion of chemical energy into electrical energy can be performed in a fuel cell (FC). Such a fuel cell uses the released energy from a chemical reaction and transforms it into electric energy. The most common setup is the proton exchange fuel cell (PEMFC) which employs the highly exothermic redoxreaction of hydrogen and oxygen to water:
H2 → 2 H+ + 2 e-
1/2 O2 + 2 H+ + 2 e- → H2O
This type of fuel cell consists of three parts: a catalyst at the andode side (hydrogen is split up into protons and electrons), another catalyst at the cathode side (where the oxygen is added), and a proton conducting membrane that separates the two sides. This membrane allows only protons to travel from one electrode to the other, while blocking the migration of all other particles.
There are some polymers that exhibit this functionality, the most widely used being Nafion(R). It consists of a hydrophobic polymer matrix with bulk water channels through the material. These water channels solvate the acidic protons from the polymer, and thus enable long-range proton transport through the entire membrane along the hydrogen-bond network of the water molecules. However, the presence of bulk water channels limits the operating temperature of a fuel cell to the boiling temperature of water. This is a big limitation, since the efficiency and lifetime of the fuel cell could be greatly increased if the temperature could be raised, and, as a consequence, the search for high-temperature membrane or separator materials is a field of intense research.
In the progress of this search, experimentalists suggested to exploit the amphoteric properties of phosphonic acids which are known to have one of the highest proton conductivities in their liquid state. Particular examples that employ phosphonic acids are PVPA[1] and p-6 PA-HPB[2]; the latter is an organic, disk-shaped molecule that self-organizes in columnar stacks. While this material showed promising proton conductivity at high temperatures and low humidities in experimental studies, the actual conduction mechanism remained unclear.
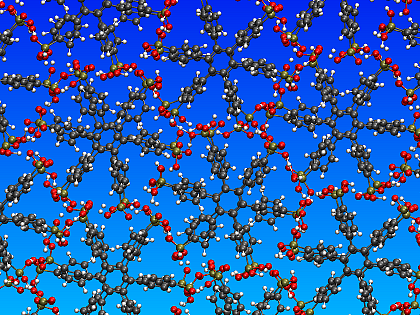
Hexagonal arrangement of the stacks of p-6PA-HPB
In this project, we investigate the structural packing of water-free p-6 PA-HPB supramolecular assemblies as well as the structural and dynamical properties of the emerging hydrogen bond network to unravel the actual conduction mechanism on the atomistic level. Because of the complexity of this problem, we employ a combination of molecular modeling and ab initio molecular dynamics simulations to brigde the scales between its individual facets. [3]
We address the question of the structural packing by means of molecular modeling with an atomistic forcfield. Our molecular dynamics simulations yield the formation of columnar stacks on a hexagonal lattice with a highly optimized space-filling arrangement and a dense but nonetheless flexible hydrogen bond network between the phosphonic acid groups.
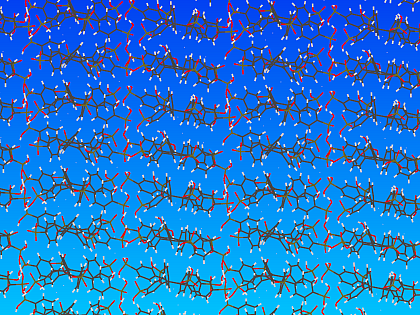
The columnar arrangement promotes proton movement along the stacks
For the modeling of the actual conduction mechanism, we employ molecular dynamics simulations based on electronic structure theory, in particlular, Kohn-Sham density functional theory. Our ab initio simulations show the formation of a strong, strictly intermolecular hydrogen bond network: at all times, each acid group is hydrogen-bonded to two of four possible neigbouring acid groups. An analysis of the hydrogen bond autocorrelation indicates a slow (0.3ps) and a fast process (3-12ps) in the dynamics of the hydrogen bond network which correspond to the reversible switching of a hydrogen bond between neighboring acceptor oxygens from different groups and the rotational motion of the phosphonic acids.
The most interesting observation in these simulations, however, was the formation of quasi-one-dimension channels in the interstice between adjacent supramolecular stacks; tracking of the motion of individual protons revealed that their diffusion is most pronounced in these channels.
Publications:
[1] Ludueña, Guillermo A and Kühne, Thomas D and Sebastiani, Daniel: Mixed Grotthuss and Vehicle Transport Mechanism in Proton Conducting Polymers from Ab initio Molecular Dynamics Simulations
[2] Jiménez-García, Lucía and Kaltbeitzel, Anke and Pisula, Wojciech and Gutmann, Jochen S and Klapper, Markus and Müllen, Klaus: Phosphonated Hexaphenylbenzene: a Crystalline Proton Conductor
[3] Wehmeyer, Christoph and Schrader, Manuel and Andrienko, Denis and Sebastiani, Daniel: Water-Free Proton Conduction in Hexakis(p-phosphonatophenyl)benzene Nano-Channels




