Intermolecular interactions - electronic polarization
When two molecules are in close proximity, they start to see each other and change their properties. This reaction of the molecules is a quantum process and determines the microscopic and macroscopic properties of matter. For example, it can make the difference between the liquid or gaseous state.
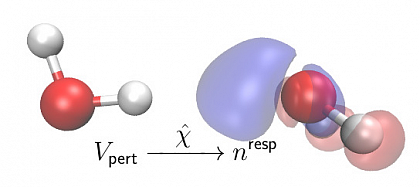
In more physical terms, when two molecules approach each other, the nuclei and electrons in both molecules start to interact with the respective other molecule. This intermolecular interaction leads to a change in the electronic orbitals and the nuclear configuration, which results in the polarization of the two molecules. Polarization effects are an important part of intermolecular interactions, e.g. in the simulation of large bio-molecules.
In this project, we develop a new efficient formalism for this kind of effects.
Our approach uses the electronic susceptibility χ to obtain the general response of the molecular electronic density for any perturbing potential. Our aim is to develop a new generation of polarizable models that is generally applicable and efficient at the same time.
Read more...
webpage-chi.pdf
(1,8 MB) vom 16.11.2016



