Structural and Dynamical Properties of Liquids under Silica Confinement
Periodically structured porous material has evoked wide interest for various applications in recent years. Experimental studies regarding the properties of liquids confined within it have been performed at length, which provides a lot of data that may be compared to simulation results.
In our simulations, models emulating the important features of MCM-41 are constructed. We investigate the anomalous structure and hydrogen bond network of liquids molecules under silica confinement. In addition to geometric data, we use proton NMR chemical shifts as a measure for the strength of the H-bonding network. We compute the ¹H NMR shifts of confined water based on a first principle approach in the framework of density functional perturbation theory under periodic boundary conditions. In our first system, two silica slabs consisting of 6 Silicide acids (Si(OH)4) are constructed and water molecules are confined in between. As shown in FIG.1.
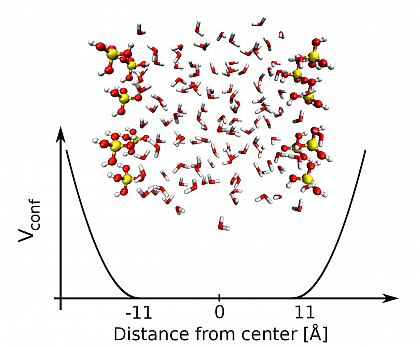
FIG.1 Two silica-water unit cell from a snapshot of the simulation
On the structural level, we see a strong influence on the ¹H chemical shifts of the confined water. While at the center of the pore a 0.5 ppm increased chemical shift compared to bulk water is found, the chemical shift gradually decreases when approaching the wall, until it reaches a value 3 ppm below bulk water reference.
This implies an enhanced hydrogen bonding network for the water at the center, and a strongly weakened network close to the silica-water interface. (FIG.2)
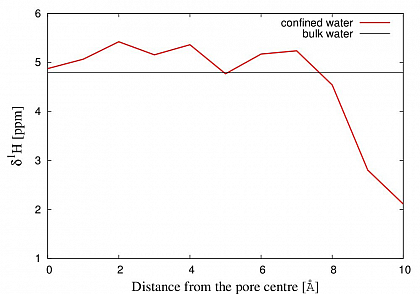
FiG.2 Calculated ¹H NMR chemical shifts of water confined between silica slabs compared with bulk water.
At the same time, we also perform DFT-MD simulations on a more realistic cylindrical pore (as shown in FIG.3)
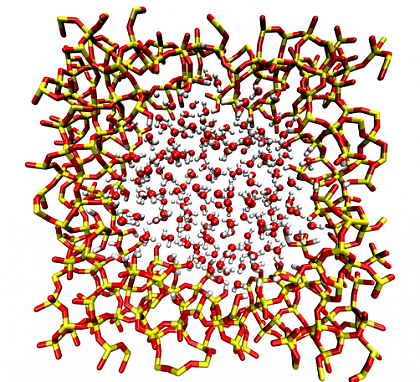
FIG.3 snapshot of the cylindrical silica pore
Furthermore, classical MD simulations of liquids mixtures under silica confinement are being carried out.
Related publications:
X.Y. Guo, T. Watermann, S. Keane, C. Allolio and D.Sebastiani:
First Principles Calculations of NMR chemical shifts of Liquid Water at an Amorphous Silica Interface
Z. Phys. Chem. 226, 1415-1424 (2012)



