Ion Specific Effects
The Hofmeister series phenomenologically quantifies the influence of ions on many phenomena such as protein precipitation or folding. The underlying ion specific effects are of fundamental importance for chemistry, liquid state physics and biology. They are often discussed in terms of ‘enhancing’ or ‘breaking’ bulk water structure. Results from THz spectroscopy, as well as time dependent infrared spectroscopy reraise the question how far the hydrogen bonding network of the bulk solution is affected by the presence of ions. For the description of the interaction between the hydrogen bonding network and ions, ab-initio methods can be used to their full advantage. As can be seen from Fig. 1, the formation of a tetrahedral coordination shell at Li+ as well as hydrogen bonding to I- arise naturally from the simulation. Due to polarizability being included, also indirect effects on hydrogen bonding, such as reinforcement by polarization of coordinated water molecules e.g. in a Li+ shell can be quantified. This effect is accessible experimentally by simple NMR chemical shift measurements. A strong relation between 1H-NMR shifts and the hydrogen bond length exists, so that it may be used to verify that a correct description of the hydrogen bond network has been obtained.
Fig. 1: Snapshot of an MD-Simulation of 1 M LiI
While insights from NMR experiments are typically constrained by the timescale of measurement, the availability of the electronic structure allows us to compute the instantaneous NMR chemical shifts. Instantaneous chemical shifts are computed from snapshots of the trajectory and can be used to decompose the experimental 1H-NMR chemical shift spectrum. The simulation of a LiI solution, therefore shows, how iodine and lithium influence proton NMR shifts in their immediate vicinity (see Fig. 2, left). Experimentally, only the bulk value is accessible - and can be reconstructed from the averages.
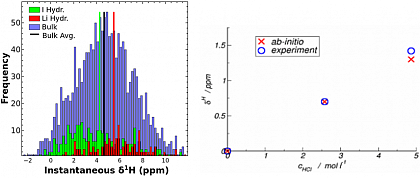
Fig. 2: Instantaneous proton NMR chemical shifts around lithium and iodide ions (left). Correlation beetween experimental and computed NMR chemical shifts in HCl solutions at different concentrations (right).
Very good agreement with experiment can be achieved in this way (see Fig. 2, right). Due to the increases in computing power, ion specific effects can now also be studied at interfaces of peptides, polymers or in confinement - with the accuracy of ab-initio simulations.
Publications:
C. Allolio, N. Salas-Illanes, Y. S. Desmukh, M. R. Hansen, D. Sebastiani
H-Bonding Competition and Clustering in Aqueous LiI
J. Phys. Chem. B, 117(34), 9939-9946(2013)
T. Murakhtina, J. Heuft, J.E. Meijer and D. Sebastiani
Ab-initio and experimental ¹H NMR signatures of solvated ions: the case of HCl(aq)
ChemPhysChem, 7, 2578-2584 (2006)



