Research
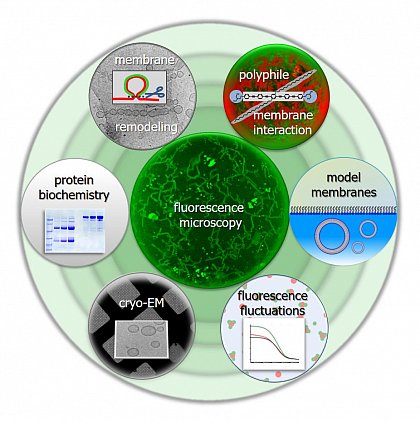
Research in the lab of Prof. Dr. Kirsten Bacia focuses on biological membranes and biophysical tools for studying proteins and membranes.
- We perform reconstitution experiments to learn how proteins remodel lipid bilayers and how proteins and synthetic polyphilic molecules interact with lipid assemblies.
- We further develop membrane models as well as microscopy-based and spectroscopy-based tools for studying these supramolecular assemblies and their interactions.
We are a member of the Institute of Chemistry, Dept. of Physical Chemistry (Faculty of Natural Sciences II). Our lab is located at the Charles-Tanford-Protein Center, together with labs of the Faculty of Natural Sciences I (Life Sciences) and the Medical Faculty, and it is part of the Centre of Innovation Competence (Zentrum für Innovationskompetenz) HALOmem .
Membrane remodelling
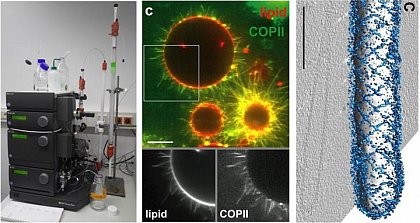
Intracellular transport requires the remodeling of lipid membranes. At the Endoplasmic Reticulum (ER) of eukaryotic cells, a set of proteins assembles into a complex, the COPII complex, which forms a protein coat around a nascent lipid membrane bud. We are interested in the physicochemical mechanisms behind this astonishing membrane remodeling process and in what is really required for the bud to proceed through bilayer fission. This a long-standing question in the field of intracellular trafficking.
Artificial membrane systems
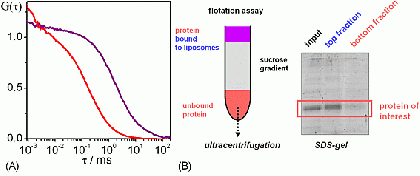
Liposomes can be prepared in various sizes, ranging from tens of nanometers to tens of micrometers. Reconstitution of integral membrane proteins into liposomes (i.e. the formation of proteoliposomes) as well as the peripheral binding of coat proteins to liposomes is analyzed using both biochemical and biophysical assays.
Very large liposomes, so-called Giant Unilamellar Vesicles (GUVs), prepared by the electroformation method are on the order of 10 µm in size, making them well-suited for studies by confocal fluorescence microscopy and fluorescence correlation spectroscopy (FCS, see below).
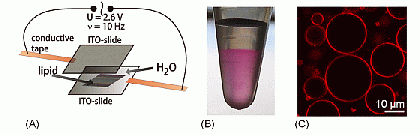
Langmuir monolayers are prepared at the buffer/air interface. The interaction of proteins with the monolayers can be studied using infrared reflection absorption spectroscopy (IRRAS).
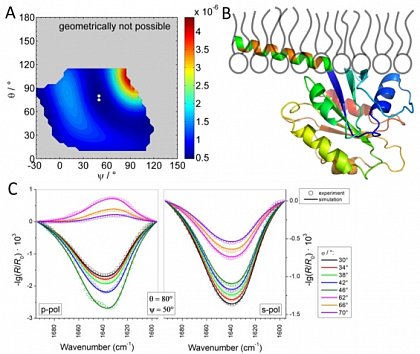
Lipid phase behavior We are interested in dynamic lateral heterogeneities in membranes from natural components (proteins, lipids) and synthetic components (artificial amphiphiles and polyphiles).
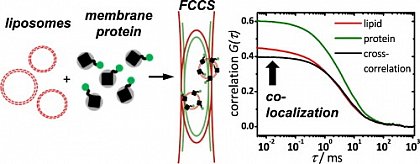
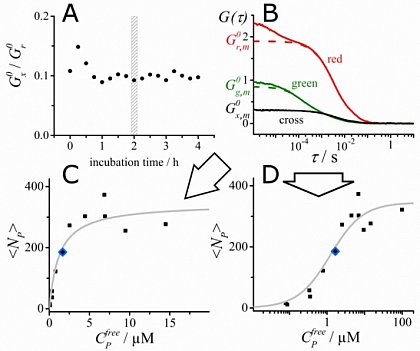
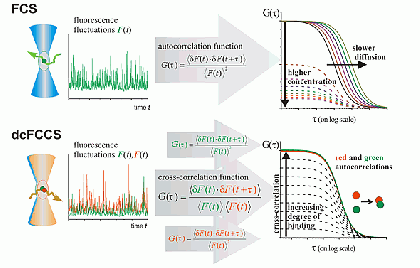
Fluorescence correlation spectroscopy (FCS) is a very useful tool for examining mobility and interactions in a variety of systems including membranes. FCS is highly sensitive to small differences in the diffusion rates of proteins and lipids, which allows for instance to characterize differences in phase behavior of lipid bilayers. FCS is used to analyze the binding of diffusible ligands to membrane receptors, such as membrane proteins or glycolipids. Changes in the fluorescence brightness parameter reveal membrane protein oligomerization. Moreover, the use of dual-color fluorescence cross-correlation (dcFCCS) allows to assess protein-protein binding in cases, where binding does not lead to significant changes in diffusion rates. The dual-color cross-correlation technique can also be employed to detect dynamic co-localization of labeled cargo molecules in small, mobile carriers, such as transport vesicles. Owing to the use of fluorescent labels, FCS is highly specific and can be applied both to artificial, reconstituted systems and directly to living cells.
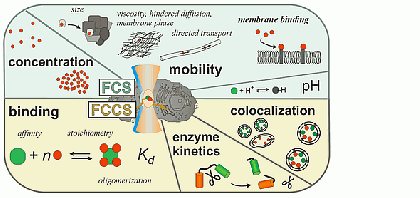
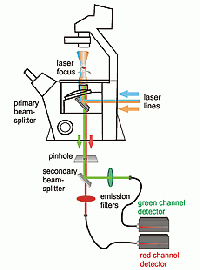
FCS is typically performed on a setup that is similar to a confocal microscope. One or more laser lines are focused in the sample and the fluorescence is collected through the same objective. A pinhole serves to delimit the detection volume. The fluorescence emission(s) from the label(s) are selected by means of emission filter(s) and the fluorescence intensity as a function of time is recorded by avalanche photodiode detectors. Different methods of analysis are available to extract information from the fluorescence fluctuations, which occur as labeled molecule diffuse through the focus. Correlation analysis yields an autocorrelation curve, whose amplitude is inversely related to the concentration of the fluorescent particles. The decay time of the correlation curve reflects the diffusional mobility of the particles. In dual-color FCCS, the relative amplitude of the cross-correlation curve depends on the fraction of double-labeled (i.e., bound) particles.
Methods and instrumentation
We are interested in working out other applications of the methods we use in the lab, e.g. in cell biology and plant biology through active collaborations. Please get in touch if any of the following spectroscopic methods are of interest for your research:
- Fluorescence Fluctuation Spectroscopy (particle diffusion/mobility analysis in solution, in model system and inside live cells using fluorescence correlation spectroscopy (FCS), binding analysis using dual-color fluorescence cross-correlation spectroscopy (FCCS), diffusion/mobility analysis using raster image correlation spectroscopy (RICS))
- Confocal Fluorescence Microscopy
- Laser Scanning Microscopy (LSM) on an Inverse or Upright Scope
- Zeiss Airy Scan (improved spatial resolution)
- Spinning Disc (fast scanning for dynamic processes)
- Confocal fluorescence microscopy on cryo-fixed samples (in liquid nitrogen)
- Confocal anisotropy imaging
- Total Internal Fluorescence (TIRF) and single molecule localization microscopy (Zeiss Elyra)
The same applies to methods for producing and characterizing model membrane systems, e.g. monolayers on a Langmuir film balance (stand-alone or combined with widefield or confocal microscopy), giant unilamellar vesicles (GUVs), membrane protein reconstitution into liposomes, flotation assays, dynamic light scattering, sample preparation for cryo-EM at HALOmem (Lab of Prof. Dr. Kastritis) or the EM facility (Dr. Stephanie Krüger) and more.
Contacts: Dr. Caroline Haupt, Dr. Sebastian Daum
Interested in joining the lab?
Being an interdisciplinary team, we welcome contributions from biology, biochemistry, chemistry, physics, medical physics, pharmacology, engineering and other relevant backgrounds.
We always welcome inquiries from students interested in a PhD project and from postdocs. Current job announcements can be found here.
Students interested in joining the lab during their Bachelor or Masters program should feel free to contact Prof. Kirsten Bacia. Periodically, listings of exemplary Bachelor thesis projects are available at the Institute of Biochemistry/Faculty of Natural Sciences I. A listing and further information can also be found at the Institute of Chemistry/Faculty of Natural Sciences II by signing up to 'Abschlussarbeiten in der Chemie' on Stud.IP.




