4. Metal Complexes with Bioactive Ligands
The discovery of the carcinostatic effect of cis-[PtCl2(NH3)2] by Rosenberg (1969) and investigations on the mechanism of action of platinum compounds in chemotherapy promoted the development of the coordination chemistry of platinum(II) with biologically important ligands. Nowadays, attention is focused on platinum(IV) complexes with bioactive ligands, because of the lower toxicity of platinum(IV) and the possibility of oral administration of some potent platinum(IV) compounds as well as the fact that they can coordinate to DNA without prior reduction to platinum(II) compounds. In general, platinum(IV)complexes with bioligands have been less thoroughly investigated than platinum(II) complexes, and in most cases they have been prepared by oxidation of the corresponding platinum(II) complexes. We investigate the synthesis of carbohydrate and amino acid complexes of platinum(IV) by ligand substitution reactions. Some results are:
- Synthesis and characterization of the first pentachloro(aqua)platinate complexes H3O[PtCl5(H2O)]·6H2O·2(18C6) (1) and [K(18C6)][PtCl5(H2O)]. In the solid state, complex 1 (Fig. 11) exhibits an unusual structure: In a cage of three crown ether molecules an [H13O6]+ cation is embedded being a new isomer of [H13O6]+.
- Complex 1 reacts with amino acids to give amino acid platinum(IV) complexes with interesting coordination modes of the amino acid ligands, see Figure 12 as an example. It has been shown that the crown ether influences the course of the ligand substitution reactions. Complex 1 also reacts with nucleobases yielding platinum(IV) complexes with protonated nucleobase ligands, see Figure 13 as an example.
- [PtMe3(Me2CO)3]BF4 reacts with carbohydrates to give the first carbohydrate platinum(IV) complexes, see Figure 14 as an example. Although the carbohydrates are only weak donors, pyranoses, furanoses, and acyclic carbohydrates can be bound to platinum(IV). The carbohydrates are neither oxidized by platinum(IV) nor deprotonated upon complexation, although the latter often occurs using metals in high oxidation states. Importantly, platinum promoted cleavage or formation of isopropylidene protection groups and Schiff bases can occur depending on steric conditions.
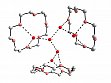
Figure 11: Structure of [H13O6]+ in a cage of three crown ether molecules 18C6. Detail of solid-state structure of H3O[PtCl5(H2O)]·6H2O·2(18C6).
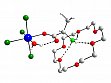
Figure 12: [PtCl4(alaH)(H2O)]·(18C6), a platinum(IV) complex with an zwitterionic amino acid ligand that is O-coordinated to platinum.
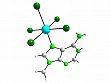
Figure 13: [PtCl5(MeAdeH)](MeAde = 9-methyladenine), a platinum(IV) complex with an N7 coordinated and N1 protonated 9-methyladeninium ligand.

Figure 14: Molecular structure of the cation in [PtMe3(ch)]BF4(ch = 1,2-O-isopropylidene-α-D-glucofuranose).



