Chirality induction
Chirality is a fundamental phenomenon which everybody can experience by considering the different "handedness" of the own hands. Virtually all biomolecules are chiral, be it as enantiomers or due to their supramolecular assemblies like in DNA helices. It is still a big mystery why life on earth features mostly one sense of chirality. In general, enantiomers have different bio-activities, the difference can be as drastic as in case of the thalidomide molecule, where chirality makes the difference between a useful drug and a teratogen.
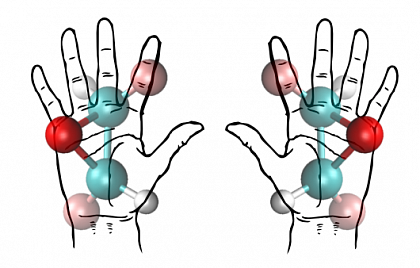
Like our hands, enantiomers are mirror images of one another.
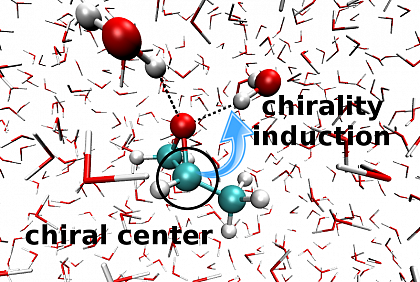
Due to the hydogen bonding, propylene oxide induces its chirality to the achiral water molecules.
Enantiomers interact differently with circularly polarized light. This allows us to determine their absolute configuration by means of optical activity spectroscopy. Most standard tools like conventional infrared-, Raman- or NMR spectroscopy are not able to distinguish between enantiomers. This is due to the fact that chiral molecules have identical properties in almost all respects (e.g. molecular mass, intra-molecular distances of the atoms, dipoles etc.).
In this project, we are interested in the mechanism by which chirality is mediated between molecules. We study these effects on an atomistic level using quantum chemical methods.
Read more...
webpage-vcd-neu.pdf
(878,5 KB) vom 16.11.2016



