Ultrafast proton transfer in aqueous media: Ab-initio molecular dynamics simulation of photoacids
Biophysical processes often take place based on proton relay along a hydrogen bonded chain. Such proton transfer reactions along ”water wires” are difficult to observe directly inside a protein. We aim to critically assess the sequential proton hopping and concerted water wire proton transport models by using quantum mechanical molecular dynamics simulations.
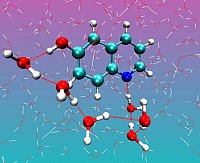
Figure 1: Snapshot from the MD trajectory of cis-6HQ with the shortest water wire
Photosensitive acid/base systems provide a method to control and study ultrafast proton transport via Infrared spectroscopy. Specifically, a particular water wire class of photoacids, hydroxyquinolones (HQ) and naphtol-derivatives deserve special attention from both fundamental and practical point of view. Hydroxyquinolones are bifunctional molecules which provide both an acidic site with proton donatiting functionality as well as a conjugate basic site that can accept a proton. After promoting the molecule to the excited state, the proton transfer reaction is initiated, which usually happens from the acidic to conjugate photobase site along the hydrogen bond network. Departing from our successful simulation of the excited state dynamics and fluorescence shift of the related N-methyl-6-quinolone, we study the ground and excited state solvation of different HQs, focussing on the identification of water wires and excited state protonation dynamics.



