Research
Research in the lab of Prof. Dr. Kirsten Bacia focuses on biological membranes and biophysical tools for studying proteins and membranes.
- We perform reconstitution experiments to learn how proteins remodel lipid bilayers and how proteins and synthetic polyphilic molecules interact with lipid assemblies.
- We further develop membrane models as well as microscopy-based and spectroscopy-based tools for studying these supramolecular assemblies and their interactions.
We are a member of the Institute of Chemistry, Dept. of Physical Chemistry (Faculty of Natural Sciences II). Our lab is located at the Charles-Tanford-Protein Center, together with labs of the Faculty of Natural Sciences I (Life Sciences) and the Medical Faculty, and it is part of the Centre of Innovation Competence (Zentrum für Innovationskompetenz) HALOmem .
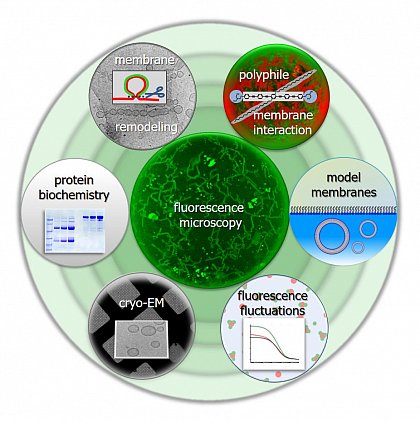
Research interests: We study lipid bilayers, interactions of proteins and synthetic molecules with membranes and proteins in solution. We apply and further develop model membrane tools and optical tools as well as new combinations thereof.
Membrane remodelling
Intracellular transport requires the remodeling of lipid membranes. At the Endoplasmic Reticulum (ER) of eukaryotic cells, a set of proteins assembles into a complex, the COPII complex, which forms a protein coat around a nascent lipid membrane bud. We are interested in the physicochemical mechanisms behind this astonishing membrane remodeling process and in what is really required for the bud to proceed through bilayer fission. This a long-standing question in the field of intracellular trafficking.
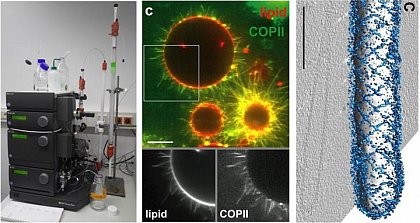
Membrane remodelling by proteins
Left: Protein purification by chromatography (ÄKTA FPLC). Middle: Confocal microscopy image of the COPII coat reconstituted on giant liposomes (Bacia et al., Sci. Rep. 2013), scale bar = 10 µm.
Right: Organization of the COPII on a lipid membrane tubule from cryo-electron microscopy (Zanetti et al., eLife 2013), scale bar = 100 nm.
Artificial membrane systems
Liposomes can be prepared in various sizes, ranging from tens of nanometers to tens of micrometers. Reconstitution of integral membrane proteins into liposomes (i.e. the formation of proteoliposomes) as well as the peripheral binding of coat proteins to liposomes is analyzed using both biochemical and biophysical assays.
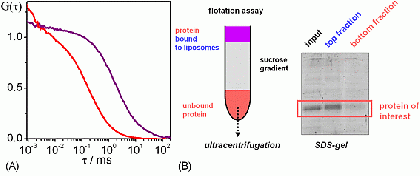
(A) FCS (fluorescence correlation spectroscopy) analysis of membrane protein reconstitution. The protein carries a fluorescent label. Free protein in solution diffuses fast (red curve), whereas proteoliposomes diffuse much more slowly (purple curve). (B) Biochemical assay for membrane protein reconstitution.
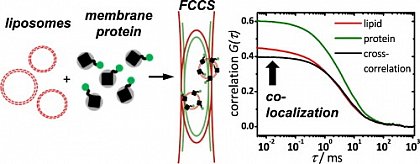
Using dual-color fluorescence cross-correlation spectroscopy (FCCS), the success of membran protein reconstitution can be assessed within minutes. (from Simeonov et al., Biophys. Chem. 2013, doi: 10.1016/j.bpc.2013.08.003)
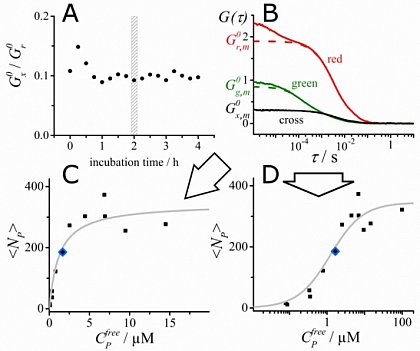
Dual-color FCCS (see below) allows to obtain quantitative binding curves of protein (or ligand) binding to freely diffusing liposomes (from Kruger et al., BioRxiv 2017, DOI 10.1101/146464 , now published in Biophys. J. 2017, doi: 10.1016/j.bpj.2017.06.023)
Very large liposomes, so-called Giant Unilamellar Vesicles (GUVs), prepared by the electroformation method are on the order of 10 µm in size, making them well-suited for studies by confocal fluorescence microscopy and fluorescence correlation spectroscopy (FCS, see below).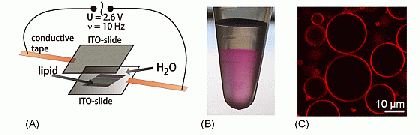
(A) Electroformation setup for producing Giant Unilamellar Vesicle; (B) Dye-labeled Giant Unilamellar Vesicles in a test tube; (C) Confocal slice image of Giant Unilamellar Vesicles
Langmuir monolayers are prepared at the buffer/air interface. The interaction of proteins with the monolayers can be studied using infrared reflection absorption spectroscopy (IRRAS).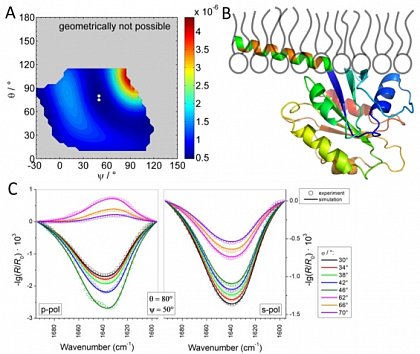
Determination of the orientation of the COPII-protein Sar1p at a lipid monolayer by IRRAS (from Schwieger et al., Polymers 2017, doi: 10.3390/polym9110612)
Lipid phase behavior We are interested in dynamic lateral heterogeneities in membranes from natural components (proteins, lipids) and synthetic components (artificial amphiphiles and polyphiles).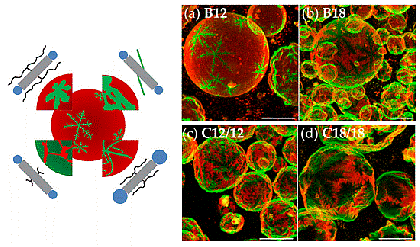
Dendritic, star-shaped domains with sixfold symmetry formed in membranes from DPPC and X-shaped bolapolyphiles (from Werner et al., Polymers 2017, doi:10.3390/polym9100476, see also Werner et al., Chem. Eur. J. 2015, doi 10.1002/chem.201405994). The molecular structure of the bolapolyphile influences the domain shape.
Fluorescence correlation spectroscopy (FCS) is a very useful tool for examining mobility and interactions in a variety of systems including membranes. FCS is highly sensitive to small differences in the diffusion rates of proteins and lipids, which allows for instance to characterize differences in phase behavior of lipid bilayers. FCS is used to analyze the binding of diffusible ligands to membrane receptors, such as membrane proteins or glycolipids. Changes in the fluorescence brightness parameter reveal membrane protein oligomerization. Moreover, the use of dual-color fluorescence cross-correlation (dcFCCS) allows to assess protein-protein binding in cases, where binding does not lead to significant changes in diffusion rates. The dual-color cross-correlation technique can also be employed to detect dynamic co-localization of labeled cargo molecules in small, mobile carriers, such as transport vesicles. Owing to the use of fluorescent labels, FCS is highly specific and can be applied both to artificial, reconstituted systems and directly to living cells.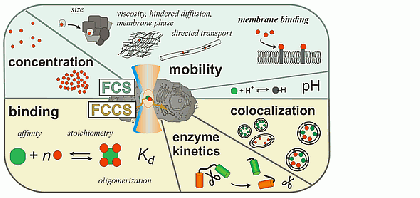
Parameters accessible by FCS (for details see: Bacia et al., Nat. Methods 2006)
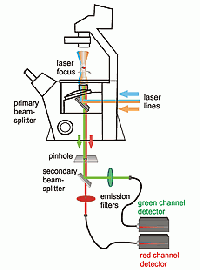
Schematic view of an FCS setup with dual color FCCS capability
FCS is typically performed on a setup that is similar to a confocal microscope. One or more laser lines are focused in the sample and the fluorescence is collected through the same objective. A pinhole serves to delimit the detection volume. The fluorescence emission(s) from the label(s) are selected by means of emission filter(s) and the fluorescence intensity as a function of time is recorded by avalanche photodiode detectors. Different methods of analysis are available to extract information from the fluorescence fluctuations, which occur as labeled molecule diffuse through the focus. Correlation analysis yields an autocorrelation curve, whose amplitude is inversely related to the concentration of the fluorescent particles. The decay time of the correlation curve reflects the diffusional mobility of the particles. In dual-color FCCS, the relative amplitude of the cross-correlation curve depends on the fraction of double-labeled (i.e., bound) particles.
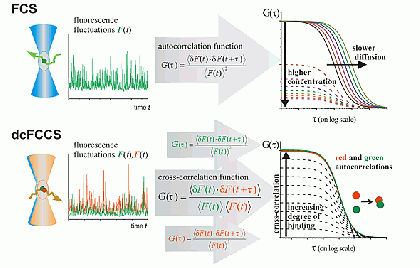
Principle of FCS and dcFCCS measurements, for details see Bacia et al., Nat. Methods 2006
Methods and instrumentation
We are interested in working out other applications of the methods we use in the lab, e.g. in cell biology and plant biology through active collaborations. Please get in touch if any of the following spectroscopic methods are of interest for your research:
- Fluorescence Fluctuation Spectroscopy (particle diffusion/mobility analysis in solution, in model system and inside live cells using fluorescence correlation spectroscopy (FCS), binding analysis using dual-color fluorescence cross-correlation spectroscopy (FCCS), diffusion/mobility analysis using raster image correlation spectroscopy (RICS))
- Confocal Fluorescence Microscopy
- Laser Scanning Microscopy (LSM) on an Inverse or Upright Scope
- Zeiss Airy Scan (improved spatial resolution)
- Spinning Disc (fast scanning for dynamic processes)
- Confocal fluorescence microscopy on cryo-fixed samples (in liquid nitrogen)
- Confocal anisotropy imaging
- Total Internal Fluorescence (TIRF) and single molecule localization microscopy (Zeiss Elyra)
The same applies to methods for producing and characterizing model membrane systems, e.g. monolayers on a Langmuir film balance (stand-alone or combined with widefield or confocal microscopy), giant unilamellar vesicles (GUVs), membrane protein reconstitution into liposomes, flotation assays, dynamic light scattering, sample preparation for cryo-EM at HALOmem (Lab of Prof. Dr. Kastritis) or the EM facility (Dr. Gerd Hause) and more.
Contacts: Dr. Caroline Haupt, Dr. Sebastian Daum
We are able to collaborate on a range of projects. For core facility type services in light microscopy, please refer to the CFI (Core Facility Imaging) of the Medical Faculty in the same building.
Interested in joining the lab?
Being an interdisciplinary team, we welcome contributions from biology, biochemistry, chemistry, physics, medical physics, pharmacology, engineering and other relevant backgrounds.
Students interested in joining the lab during their Bachelor or Masters program should feel free to contact Prof. Kirsten Bacia. Periodically, listings of exemplary Bachelor thesis projects are available at the Institute of Biochemistry/Faculty of Natural Sciences I and the Institute of Chemistry/Faculty of Natural Sciences II, respectively.
Students interested in a PhD project and Postdocs are also welcome to inquire about opportunities.
Job announcements at the University of Halle can be found at the Human Resource Department.
