
Kontakt
Prof. Dr. Marcus A. Glomb
Telefon: 0345 55 25784
Telefax: 0345 55 27341
marcus.glomb@chemie.uni-hal...
Raum 427
Kurt-Mothes-Str. 2
06120 Halle (Saale)
AK Prof. Glomb

Journal of Agricultural and Food Chemistry
Glycation Alters the Fatty Acid Binding Capacity of Human Serum Albumin
Henning C, Stübner C, Arabi SH, Reichenwallner J, Hinderberger D, Fiedler R, Girndt M, Di Sanzo S, Ori A, Glomb MA (2022) J. Agric. Food Chem. 70 (9), 3033-3046, DOI: 10.1021/acs.jafc.1c07218
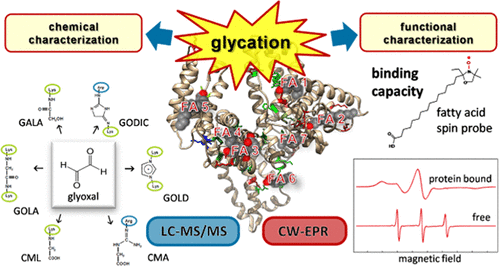
Glycation significantly alters the physicochemical and biofunctional properties of proteins in foods and in vivo. In the present study, human serum albumin (HSA) as the major transporter of fatty acids was modified with glyoxal under physiological conditions. Reversibly albumin-bound glyoxal was removed, and advanced glycation end products were quantitated by liquid chromatography–tandem mass spectrometry (LC-MS/MS). The total modification of protein-bound lysine and arginine residues reached up to 4.2 and 9.6%, respectively. The impact of these modifications on the transport capacity of long-chain fatty acids was characterized by spin-labeled fatty acid probes via electron paramagnetic resonance spectroscopy. With increasing degree of glycation, the equivalence of the seven binding sites of native HSA with a dissociation constant of 0.74 ± 0.09 μM was set off with only the three high-affinity sites 2, 4, and 5 remaining (0.46 ± 0.07 μM). The other four sites were shifted to low affinities with significantly higher dissociation constants (1.32 ± 0.35 μM). Tryptic peptide mapping enabled us to relate these findings to molecular changes at specific binding sites. Modification hotspots identified were lysine 351, 286, 159 and arginine 144, 485, 117. Further investigation of plasma protein samples of uremic patients vs healthy controls gave first insights into the in vivo situation. (Copyright ©2022 American Chemical Society)
Vortragsveranstaltung anlässlich des Weltverbrauchertages 2019

Anlässlich des Weltverbrauchertages findet am 15. März 2019 im Hörsaal B des Melanchtonaniums (Universitätsplatz) in Halle (Saale) ein gemeinsamer Vortragsnachmittag der Universität Halle, der Verbraucherzentrale Sachsen-Anhalt und des Landesamtes für Verbraucherschutz (LAV) statt. Am 16. März 2019 lädt der Fachbereich Lebensmittelsicherheit des LAV von 10 bis 14 Uhr zum „Tag der offenen Labore“ in die Freiimfelder Straße 68 in Halle ein. Das Programm entnehmen Sie bitte dem Flyer .
Journal of Agricultural and Food Chemistry
Analysis and Chemistry of Novel Protein Oxidation Markers in Vivo
Henning C, Liehr K, Girndt M, Ulrich C, Glomb MA (2018) J. Agric. Food Chem. 66 (18), 4692-4701, DOI: 10.1021/acs.jafc.8b00558
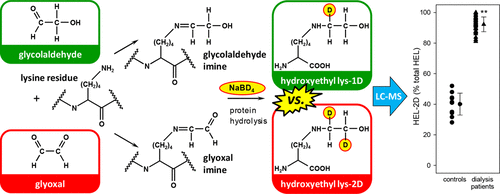
Proteins continually undergo spontaneous oxidation reactions, which lead to changes in structure and function. The quantitative assessment of protein oxidation adducts provides information on the level of exposure to reactive precursor compounds with a high oxidizing potential and reactive oxygen species (ROS). In the present work, we introduce N6-(2-hydroxyethyl)lysine as a novel marker based on the ratio of glycolaldehyde and its oxidized form glyoxal. The high analytical potential was proven with a first set of patients undergoing hemodialysis versus healthy controls, in comparison with well-established parameters for oxidative stress. In vitro experiments with N1-t-BOC-lysine and N1-t-BOC-arginine enlightened the mechanistic relationship of glycolaldehyde and glyoxal. Oxidation was strongly dependent on the catalytic action of the ε-amino moiety of lysine. Investigations on the formation of N6-carboxymethyl lysine revealed glycolaldehyde-imine as the more reactive precursor, even though an additional oxidative step is required. As a result, a novel and very effective alternative mechanism was unraveled. (Copyright © 2018 American Chemical Society)
Markus Fischer, Marcus Glomb (Hrsg.)
Moderne Lebensmittelchemie

1. Auflage 2015
Lehrbuch: 788 Seiten
Behr´s Verlag
Sprache: Deutsch
ISBN: 978-3-89947-864-8
Sie können das Buch über den Online-Shop des Behr's Verlages bestellen.
Angewandte Chemie
Maillard Degradation Pathways of Vitamin C
Selbst 80 Jahre nachdem die Struktur von L-threo-Ascorbinsäure (Vitamin C) aufgeklärt wurde, ist die Chemie dieses reaktiven reduzierenden Kohlenhydrats immer noch aufregend. In ihrer Zuschrift (DOI: 10.1002/ange.201300399 ) berichten M. A. Glomb und M. Smuda über ihre Untersuchungen zu den Maillard-Abbauwegen, die über drei Hauptfragmentierungs-mechanismen zu Carbonyl- und Dicarbonylverbindungen, Carbonsäuren oder Amid-Endprodukten führen.










