1. Heteroatom Influence in Organometallics
Investigations on the influence of heteroatoms in alkyl and vinyl metal complexes, organometallic compounds of the type LxM-(CH2)m-YRn, LxM-CH=CHYRn and LxM-C(YRn)=CH2 (L = ligand, M = metal, Y = heteroatom group 14-17, R = alkyl, aryl, H) may be characterized by an extraordinary stability and/or reactivity depending on the complex type, the distance (m) between M and Y and the nature of the heteroatom which can be (i) neutral and coordinatively saturated (YRn = SiR3, ...), (ii) neutral but Lewis-basic (YRn = NR2, PR2, OR, SR, Cl, ...) or (iii) cationic (Y+Rn = P+R3, S+R2, ...). Especially complexes of type ii attract attention because the Lewis-basic heteroatom is highly reactive in many cases and may open up the possibility of entirely new structures (e.g. η2-coordination) and reactions (e.g. carbenoid reactions). This is especially pronounced in homoleptic complexes due to the absence of any co-ligands L. We are interested in compounds of type (ii) with a geminal or vicinal arrangement of M and Y (m = 1, 2). Some results are:
- Synthesis and structural characterization of functionalized methyl lithium, magnesium, and aluminium complexes [LiCH2YRn(thf)x](YRn = NR2, PR2, SR), [Mg(CH2SR)2(thf)3], [Al(CH2SR)3]. Due to coordination of a Lewis-basic heteroatom Y to the metal, they exhibited in the solid state a high structural diversity. There were found monomeric compounds (M = Mg), dimeric compounds with four-membered Li2C2 and six-membered M2C2Y2 (M = Li, Al) rings, polymeric ladder-like structures (M = Li), polymeric structures with a spiro center (M = Al), and tetrameric (tetrahedral and non-tetrahedral) structures.
- The monomeric complex [LiCH2SPh(pmdta)] pmdta = ,N,N´,N´,N´´,N´´-pentamethyldiethylenetriamine) (see Fig. 1) reacts in toluene as a carbenoid with formation of [LiSPh(pmdta)] and methane and ethylene (dimerizing α-elimination), respectively.
- Synthesis and characterization of homoleptic aminomethyl complexes of 3d-elements [LixM(CH2NR2)y](M = Ti...Ni). [{Li(Et2O)}2Ni(CH2StBu)4] and [{Li(thf)}2Cr2(CH2PMe2)6] (Fig. 2) have been characterized as the first homoleptic mercaptomethyl complex of a 3d-element and of the first homoleptic phosphinomethyl complex of a transition metal, respectively.
- ω-Monofluoroalkylrhodoximes [Rh{(CH2)nF}(dmgH)2(PPh3)](dmgH2 = dimethylglyoxime) with n = 1 and n = 3 were prepared and structurally characterized. The synthesis of the corresponding 2-fluoroethyl complex (n = 2) failed; an unusual C-F activation resulted in formation of the dinuclear dimethylene bridged complex (PPh3)(dmgH)2Rh-CH2-CH2-Rh(dmgH)2(PPh3). Furthermore, we succeeded to synthesize and structurally to characterize the complex [Co(C6H11F)(dmgH)2{4-(t-Bu)py}](C6H11F = 2-fluorocyclohexyl) (see Fig. 3)
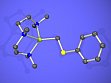
Figure 1: Structure of the monomeric compound [LiCH2SPh(pmdta)] that exhibits a carbenoid reactivity.
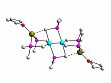
Figure 2: Molecular structure of [{Li(thf)}2Cr2
(CH2PMe2)6], a homoleptic phosphinomethyl complex of a transition metal.
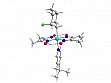
Figure 3: Structure of the 2-fluorocyclohexyl cobaloxime complex, the first structurally characterized 2-monofluoroalkyl complex.



